Medical sterilization tests
Regular sterilization monitoring is one of the fundamental and mandatory elements of sterilization procedures – regardless of the size of the facility where these processes are carried out. Recommendations regarding verification methods are described, among others, in official guidelines for facilities performing sterilization. The authors of these documents emphasize the obligatory nature of sterilization control and specify which parameters of individual sterilization methods must be verified. In the most common method, steam sterilization, this includes confirmation of the required time, temperature, pressure and the presence of steam. In hydrogen peroxide sterilization, the parameters to be controlled are time, temperature and the concentration of the sterilizing agent. The choice of testing method and the scope of sterilization control depend on the sterilization method used, as well as on the type and intended use of the sterilized material. In some particularly demanding cases (e.g. implant sterilization), the use of the most accurate biological tests is required.
Sterilization process monitoring using different tests
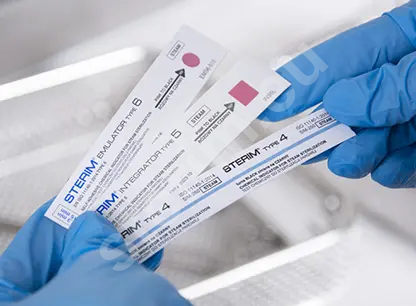
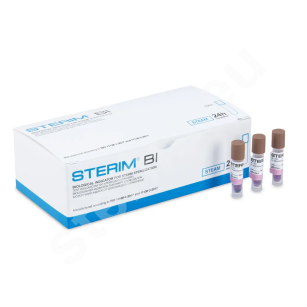
The basic and most commonly used method for verifying process parameters is chemical sterilization indicators. The indicator substance placed on the test changes when the expected process parameters are reached. Ampoule biological tests are more precise and allow verification of the actual biocidal effectiveness against biological material, i.e. selected bacterial strains. Biological tests are used to monitor sterilization in cases requiring particularly strict control and for periodic validation of sterilization processes. It is important to remember the obligation to keep documentation confirming compliance with procedures for every sterilization cycle performed at the facility. To facilitate archiving, some chemical indicator strips are equipped with an adhesive layer, making them easy to attach in logbooks. In addition to chemical indicator strips and biological ampoules, Type 1 process indicators are also used in sterilization procedures. These confirm that a package has been exposed to the sterilization process and are placed directly on sterilization packaging or on labels attached to the packs.
Autoclave tests
An autoclave and a steam sterilizer are the same device – both terms are used interchangeably in everyday language. Therefore, sterilization tests and autoclave tests refer to the same products. Just make sure that the tests you choose monitor the sterilization method used in your facility or practice. The sterim.eu offer includes tests for Enbio autoclaves, which are gaining increasing popularity on the market. A clear color change of the indicator confirms a correctly performed sterilization process. Any incorrectly developed autoclave test is a basis for repeating the sterilization cycle. If the problem occurs again, an authorized service technician should be called and the device should not be used until the fault is resolved.
Autoclave sterilization tests that will support your daily work include:
- sterilization labels with Type 1 process indicators for steam sterilization,
- Type 2 Bowie-Dick tests,
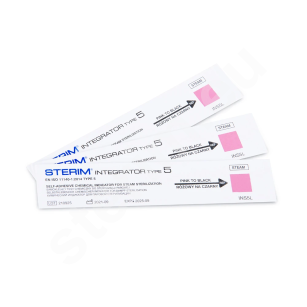
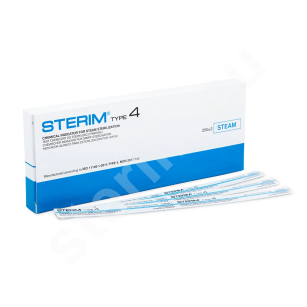
- Type 4, 5 and 6 chemical indicator strips with different sensitivities to sterilizing agents,
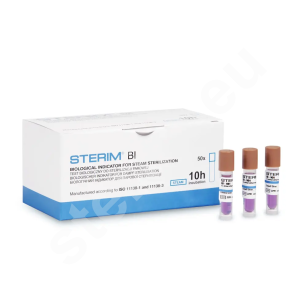
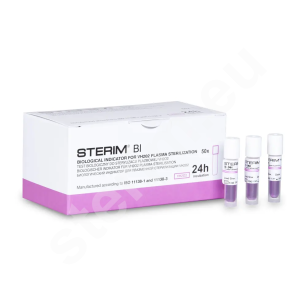
- biological spore strip tests as well as 24-hour and 10-hour ampoule biological tests,
- Helix tests for verifying steam penetration effectiveness in hollow load devices,
- accessories such as indicator sterilization tapes with Type 1 indicators.
Which tests for Enbio autoclaves?
Tests for Enbio autoclaves are the same tests used in other sterilizers with similar dimensions and operating parameters. Therefore, use tests that properly monitor your device and chemical indicator tests such as Sterim Type 4 indicators. Biological tests available at sterim.eu are also suitable for Enbio autoclaves. Autoclave tests are a reliable method of monitoring the sterilization process and can be easily archived in the required documentation, which may be inspected by sanitary authorities or other regulatory bodies in case of suspected irregularities. Sterim tests are parameter-matched to Enbio autoclaves, including their programs and sterilization cycle times. If you have difficulty choosing the right autoclave tests, contact our customer service team for professional advice.
How often and how many sterilization tests should be used?
Chemical indicator tests used to monitor the sterilization process must be applied during every sterilization cycle and should be placed in each pack sterilized in the autoclave. After removing the sterilized instruments, the test should be archived in the appropriate sterilization documentation. Biological tests, on the other hand, should be performed in accordance with the recommendations of the local sanitary and epidemiological authority – most commonly, biological testing is recommended once per week.
















 Your order will be processed
Your order will be processed 